Dr. Rudy Schlaf's Group_____________________________________________________
Department of Electrical Engineering - University of South Florida
Last Modified Aug 2013
(c) Rudy Schlaf
Webmaster
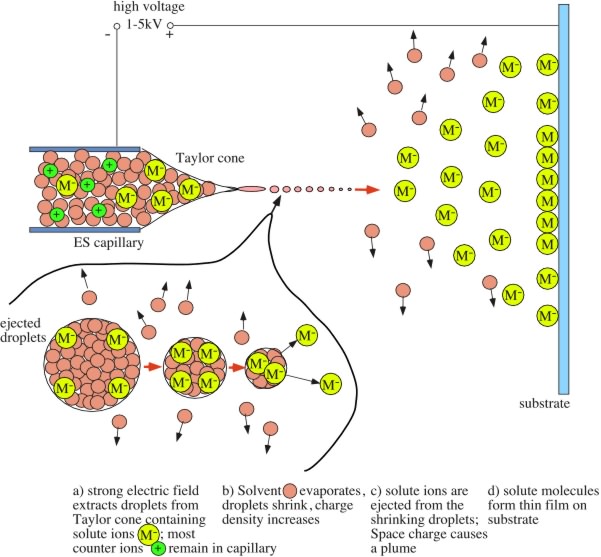
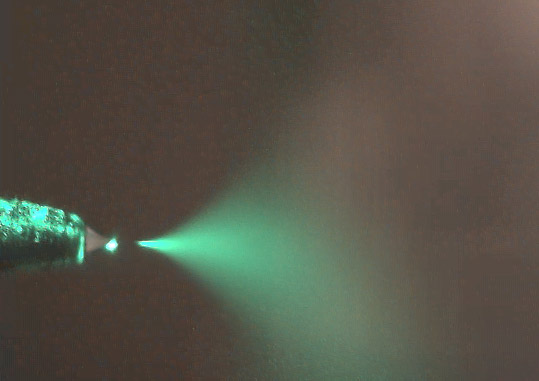
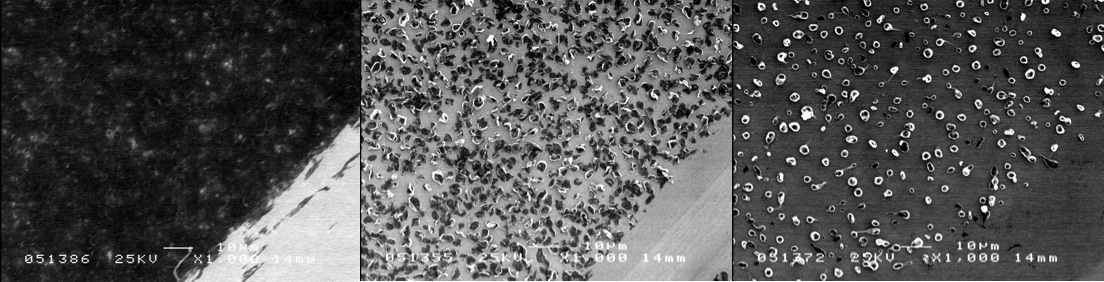
Electrospray Thin Film Deposition of Macro-Molecular Materials Under Ambient Pressure: Macro-molecular materials have become a major focus of research in recent decades. This is motivated by the promise of device applications such as plastic electronics, displays based on organic light emitting diodes, bio-sensors, or surface coating uses like controlled drug release, corrosion protection, anti-fouling coatings, or anti-static protection. Many of these applications are based on the deposition of well-defined thin film structures of macro-molecular materials on a wide range of substrates. Since macro-molecular materials are usually best processed from solution, preparative techniques such as spin coating, spraying, dipping or ink-jet printing are currently popular for thin film preparation. Electrospray combines the advantages of spray processing (fast, roll-to-roll capable, continuous) with an expanded parameter space due to electrostatic phenomena. This can be used to influence the structure of the deposited thin films. For example, Coulomb repulsion of solute ions during the spray process strongly influences the thin film structure. Hence, modification of the solvent environment can be used to achieve specific morphology goals. While electrospray has been thoroughly investigated as a technique for the introduction of ionized large molecules into vacuum for mass-spectrometry applications (electrospray ionization mass spectrometry (ESI-MS)), its use as a thin film deposition technique is a relatively unexplored territory. Our efforts currently focus on the investigation of the interplay between solution parameters (composition, concentration, solutes, solvent type etc...), spray parameters (electrical field, distance, flow rate etc...) and resulting thin film morphology. Fig.1 shows a schematic of the electrospray deposition process (see caption for details). Fig.2 shows a photograph of the electrospray plume during operation. As an example for various morphologies that can be produced by electrospray by variation of spray parameters Fig.3 shows a series of scanning electron microscopy (SEM) images taken on polylactic acid thin films prepared from dichloromethane solution at various concentrations but otherwise constant parameters. The morphology of the films ranges from strongly clustered (right) to flat thin films (left) as the concentration is reduced from 20 mg/ml to 0.5 mg/ml. Our group is currently investigating the electrospray deposition of materials ranging from various bio-molecules to polyelectrolytes and nanoparticles. |
Fig.1: Electrospray deposition process: Solution is fed through a capillary, which is positioned about 2-4 cm in front of the substrate. A high voltage applied between capillary and sample results in the formation of a Taylor cone, where a separation of positive and negative ions occurs. Droplets containing solvent and mainly ions of bias-matched polarity are emitted. As the solvent evaporates the charge density on the droplets increases, causing droplet splitting and ion ejection. Repulsive forces between the ions and droplets cause the formation of a wide plume. Once the sprayed material hits the sample surface a film is formed and charge neutralization occurs.
Fig.2: Electrospray plume illuminated with a green laser. Space charge widens the electrospray to a plume (substrate is out of focus). |
Fig.3: Polylactic acid (PLA) thin film morphology depending on PLA concentration of sprayed solution (0.5 mg/ml on left to 20 mg/ml on right). |
|